June 28, 2021
An Alternative to Aducanumab

In a desperate effort to help the over 6 million Americans living with Alzheimer’s, the only leading cause of death with no effective treatment, the FDA has recently approved a new drug, aducanumab (branded as Aduhelm), for the first time in eighteen years. While some in the community cheered the addition of a new treatment option, many clinicians and researchers were dismayed because the aducanumab trial showed the treatment had a 40% risk of brain swelling, a 17% risk of brain hemorrhaging, and did not improve memory.
Clinicians are excited about another recent Alzheimer’s development, though: based upon the early results of a clinical trial posted in medRxiv, the Yale-backed pre-print server for health sciences, in a paper entitled Precision Medicine Approach to Alzheimer’s Disease: Successful Proof-of-Concept Trial. This trial builds upon decades of laboratory and clinical work from Dr. Dale Bredesen and demonstrates the first improvements in early-stage Alzheimer’s patients. There is something else to be excited about too, while the journey from science to practice often takes decades, this intervention is already available to consumers through Apollo Health’s ReCODE Protocol ™.
Unlike Aduhelm, which requires monthly infusions and regular imaging to monitor for dangerous side effects, and which is expected to cost more than $56,000 per year, the ReCODE Protocol uses a precision medicine approach to identify and target the specific drivers of Alzheimer’s or pre-Alzheimer’s in each patient. Those specific drivers are coupled with dietary and lifestyle interventions to actually improve cognition rather than simply slowing the rate of decline. In the recent clinical trial, this led to 84% of patients improving cognition.
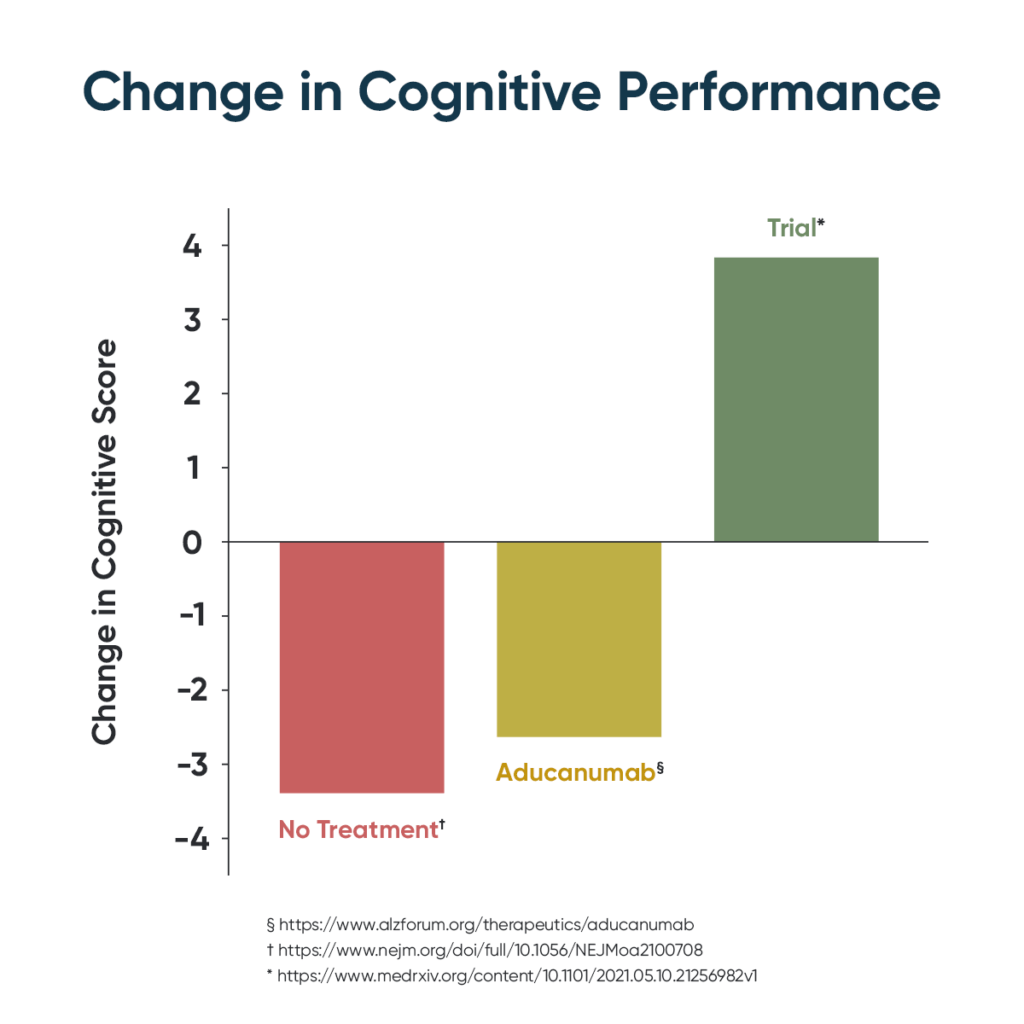
Instead of brain swelling and hemorrhaging, the only side effect of the protocol is improved health. Indeed, many participants not only optimize cognition but also optimize overall health and can discontinue prescription medications, including statins, diabetes, and hypertension drugs. The average cost to follow the protocol is far less than Aducanumab — less than $10k for the first year, with further savings from other health benefits, discontinuing prescriptions, avoiding long-term care, and even maintaining the ability to continue working. Costs also drop as healing progresses so that by the second year, it is less than 10% of the cost of Aduhelm. The strongly positive results from this clinical trial support the view that identifying and treating the contributors to cognitive decline for each patient, with a personalized protocol, represents an effective treatment approach for patients with mild cognitive impairment (MCI) or early dementia. The paper concluded that a larger, randomized controlled trial is warranted, and this follow-up study is already in the works.
The approval of Aduhelm by the FDA, despite its enormous cost, risks to health, and minimal efficacy, speaks to the desperation in the Alzheimer’s community. However, there is an alternative — the ReCODE Protocol — that offers genuine hope to the millions of people with dementia or MCI, and those at risk due to family histories of dementia, at a much lower cost, with much greater efficacy, and without dangerous side effects. If widely adopted, this will not only improve cognition and dramatically reduce costs to the entire healthcare system by optimizing overall health and addressing the chronic diseases that lead to Alzheimer’s.
You can access the ReCODE Protocol through Apollo Health, a medical technology company that offers services throughout the United States and many international locations.




