June 9, 2021
Aducanumab vs. the ReCODE Protocol

For the first time in 18 years, the FDA has approved a major Alzheimer’s drug leading some to wonder if it’s time to celebrate? Biogen’s aducanumab, now marketed as Aduhelm, did not improve cognition but did demonstrate a reduction in beta-amyloid and only minimal “success” in slowing the rate of cognitive decline in one trial at one dose after a collaboration between the FDA and Biogen to re-analyze the failed data from previous trials. This cozy relationship between a regulatory agency and large pharmaceutical has come into question, especially after the FDA overruled its own advisory committee, which overwhelmingly recommended against approving the drug.
The small potential benefit from Aduhelm comes at a huge risk and cost. Forty percent of users experienced a significant side effect of brain swelling, and 17% experienced cerebral hemorrhage. The drug is administered intravenously in a hospital setting at the price of $56,000 per year and requires a diagnostic amyloid PET scan or spinal tap as well as baseline and follow-up quarterly MRIs throughout the treatment period — all of which are expensive. Health insurance is expected to foot some of that bill, and Medicare likely will soon help to pick up the tab, which will impact U.S. taxpayers.
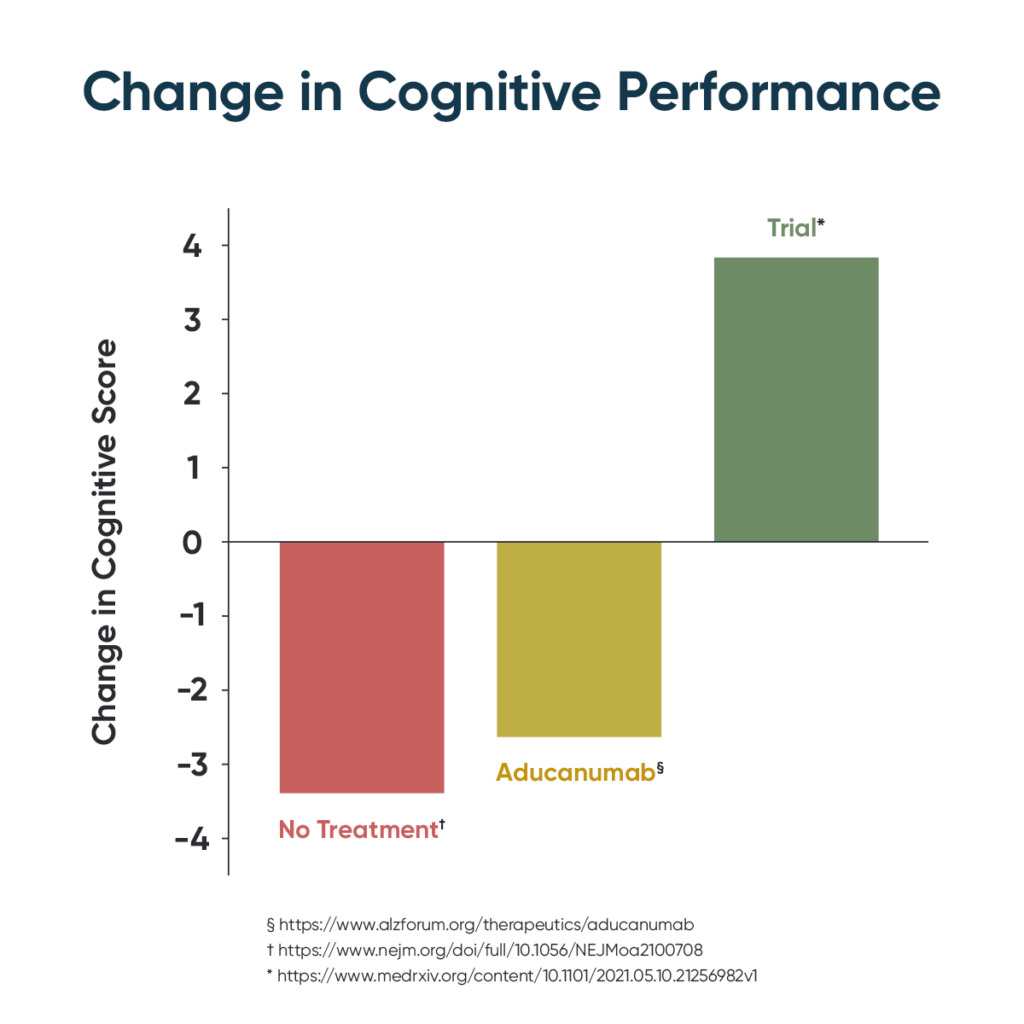
Let’s compare Aduhelm to the ReCODE Protocol, which in a recent clinical trial demonstrated 84% of participants improved cognition. Let that soak in; that’s a statistically significant improvement compared to a potential slowing of decline. Contrasted with dangerous brain swelling and hemorrhaging, the only side effect of the protocol is improved health. Indeed, many participants not only optimize cognition, but also optimize overall health and are able to discontinue prescription medications, including statins, diabetes, and hypertension drugs. The average cost to follow the protocol is less than $10k for the first year, with costs dropping as healing progresses.
When you take a hard look at the evidence behind Aduhelm’s approval process, efficacy, risks, and costs, it becomes abundantly clear that the only ones who should truly celebrate are Biogen and their stockholders. The losers are two-fold. First, the American taxpayers, who foot the bill for Medicare while paying ever-rising health insurance premiums and Alzheimer’s patients themselves, who will be at risk for significant side effects in exchange for a minor slow-down of decline. This is in sharp contrast to the significantly less expensive ReCODE Protocol, which offers statistically significant improvements in cognition as well as benefits to overall health.
If the FDA truly was interested in patient welfare, it’s clear that ReCODE should be prioritized over a pharmaceutical that was deemed futile in multiple studies and required the FDA to intervene with a new statistical analysis to find a potential benefit. Given that the FDA is charged with protecting patients from potentially dangerous and ineffective pharmaceuticals, it’s certainly time to ask the question — who’s regulating the regulators?




