March 23, 2022
ReCODE: More Than Just a Drug for Alzheimer’s Disease

By Ram Rao, Ph.D., Principal Research Scientist for Apollo Health
Last year, the US-FDA granted an ‘accelerated approval’ of the drug Aduhelm despite poor clinical trial data. Aduhelm, the formal name for Aducanumab, clears the accumulation of beta-amyloid protein from the brain. Accumulation of this protein is thought to be associated with Alzheimer’s disease (AD). However, clinical trials of the drug failed to show any significant reversal of the actual symptoms of AD. The design and mechanistic action of Aduhelm and other failed drugs for AD are based on a misconception that removing the amyloid would ameliorate AD symptoms. Unfortunately, this line of thinking is completely incorrect, and the various amyloid removing drugs — including donanemab, solanezumab, bapineuzumab, and now Aducanumab — actually supported the misconception even though the amyloid got reduced in most cases, cognition and memory were not improved. In short, Aducanumab and other failed drugs are more of an amyloid-reducing drug and less of an AD-modifying drug.
Additionally, other classes of drugs for AD that are presently in clinical trials are designed to target another protein called tau and prevent the “seeding” of tau protein to different areas of the brain, a process that helps the protein accumulate to toxic levels as tangles. The toxic spread of the tau tangles through the brain forecasts the death of brain tissue and cognitive decline. Clinical trials are now in progress to test if targeting tau and preventing its spread can slow or stop the progression of AD. However, this approach is not optimal as it is a mono-therapeutic strategy, and past single target-based failures have cast doubt on this approach.
While the anti-amyloid and anti-tau drugs showed early promise, all eventually failed, leaving more than five million Americans who suffer from AD with fewer options. Undoubtedly, the approach taken to drug development for AD is not an optimal one. And the reason is clear: genetic and biochemical research studies have revealed an extensive network of molecular interactions involved in AD development. These include, in addition to amyloid and tau, the gut microbiome, inflammatory mediators, apolipoproteins and lipid metabolism factors, vitamins, minerals, metabolic factors, hormonal mediators, neurotrophic molecules, neurotransmitters, and a host of other potential targets.
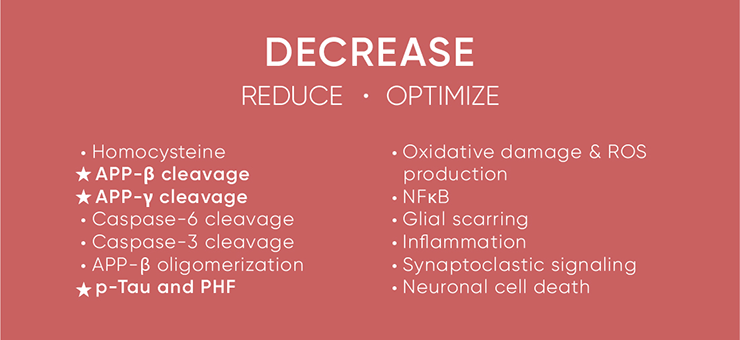
The tables below capture it all as they indicate the criteria for a perfect AD therapeutic. For effective treatment or reversal of AD, the parameters on the first table have to increase to optimal levels. In contrast, the components on the second table have to be reduced to optimal levels. Note that all the failed drugs addressed just one or two components in that vast list, as indicated by the highlighted items, which are targets of preference for most pharma and biotech companies. While several companies have decided to abandon the amyloid route and focus on tau, this mono-therapeutic approach again is not optimal and unlikely to succeed.
The most pragmatic approach would involve addressing these multiple targets underlying AD pathophysiology simultaneously; in other words, a global network-based, multi-therapeutic approach rather than a single target-based approach may be feasible and potentially more effective for AD treatment.

ApolloHealth’s ReCODE Program™ is much larger than the standard-of-care dementia evaluation data set. It identifies the various contributors to cognitive decline, such as gut dysbiosis, toxins, sleep disorders, hormonal deficiencies, withdrawal of trophic support, pathogens, and other potential contributors. The ReCODE protocol is broken down into seven distinct yet complementary strategies (The Bredesen Seven-B7) that allow the brain to heal, grow new neurons and synaptic connections, and be more resilient. While each of the B7 components is a stand-alone strategy to promote brain health, they create a powerful synergy when practiced together.
The approach we have pioneered with the ReCODE program is the future of medicine: identifying the underlying contributors to cognitive decline and targeting those specifically, rather than attempting to treat with an ineffective mono-therapeutic unrelated to the cause of the cognitive decline. The ReCODE Protocol has led to unprecedented success in treating AD, as evidenced by the over 1,000 participants who’ve experienced improvements in their cognition. Furthermore, the recent successful clinical trial, together with the several observational studies on human participants using the ReCODE protocol serves as a testimony to the program’s success.



